四类中和滴定的电导率曲线规律
时间:2020-03-11 10:00 来源:未知 作者:张安荣 点击: 次 所属专题: 中和滴定曲线 电导率曲线
版权申明:凡是署名为“化学自习室”,意味着未能联系到原作者,请原作者看到后与我联系(邮箱:79248376@qq.com)!
【电导率的意义】
电导率是用来描述物质中电荷流动难易程度的参数。高中阶段可从导电性的角度来理解:
(1)当溶液中离子浓度越大时,溶液电导率越大;
(2)当溶质离子浓度相同时,离子所带电荷越大时,溶液电导率越大。
同浓度的一元酸碱中和时,电导率大小规律:
(1)强里加强,先变弱后变强;
在0.1mol/L HCl里加入0.1mol/L 的NaOH溶液

【解读】H++ OH-= H2O
在HCl里加入NaOH,H+和OH-中和生成H2O,剩余Na+,Cl-,离子总数不变,但溶液体积增大,故离子浓度减小,电导率减小。M点到达滴定终点,运用极限思维,无限加入NaOH溶液时,相当于NaOH的电导率,所以电导率又逐渐增大。
(2)弱里加弱,先变强后变弱;
0.1mol/L NH3·H2O里加0.1mol/L CH3COOH溶液:
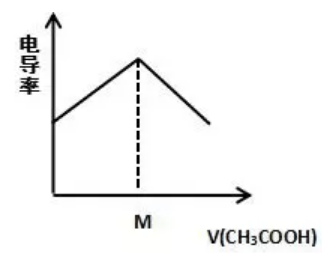
【解读】
NH3·H2O+CH3COOH = NH4++CH3COO-+H2O
原溶液弱电解质NH3·H2O中电离生成的NH4+、OH-离子浓度很小,电离程度大约1%左右,所以电导率很小;加入醋酸反应后生成强电解质,完全电离,所以溶液中离子浓度增大,电导率增大。M点到达滴定终点,无限加入CH3COOH溶液时,运用极限思维,相当于CH3COOH的电导率,所以电导率又逐渐减小。
(3)强里加弱,越来越弱;
在0.1mol/L NaOH里加0.1mol/L CH3COOH溶液

【解读】
H-+CH3COOH=H2O+CH3COO-
在NaOH里加入CH3COOH,CH3COOH中和了OH-生成H2O,离子总数目不变,但溶液体积增大,故离子浓度减小,电导率减小。M点到达滴定终点,运用极限思维,无限加入CH3COOH溶液时,相当于CH3COOH的电导率,所以电导率继续减小。
(4)弱里加强,越来越强。
在0.1mol/L 的CH3COOH里加入0.1mol/L 的NaOH
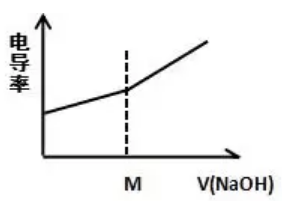
【解读】CH3COOH+OH-=H2O+CH3COO-
CH3COOH难电离,其电离生成的CH3COO-和H+浓度很小,故其电导率很小;加入NaOH后生成的CH3COONa是强电解质,离子浓度增大,故电导率增大。m(点到达滴定终点),运用极限思维,无限加入NaOH溶液时,相当于NaOH的电导率,所以电导率又逐渐增大。
【典例1】用0.1mol•L-1NaOH溶液滴定10mL0·1mol•L-1盐酸过程中的电导率曲线.下列说法错误的是( )
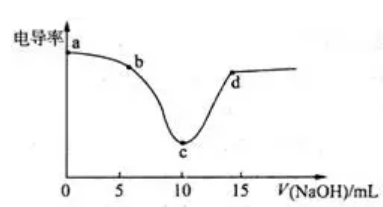
A. 电导率传感器能用于判断酸碱中和滴定的终点
B. 该过程中,a点所示溶液的导电性最强
C. C点电导率最小是因为此时溶液中导电微粒的数目最少
D. D点所示溶液中存在:C(Cl-)+c(OH-)=c(H+)+c(Na+)
参考答案:C
【典例2】(2008年广东)电导率是衡量电解质溶液导电能力大小的物理量,根据溶液电导率变化可以确定滴定反应的终点。右图是KOH溶液分别滴定HCl溶液和CH3COOH溶液的滴定曲线示意图。
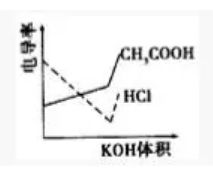
下列示意图中,能正确表示用NH3·H2O溶液滴定HCl和CH3COOH混合溶液的滴定曲线的是( )
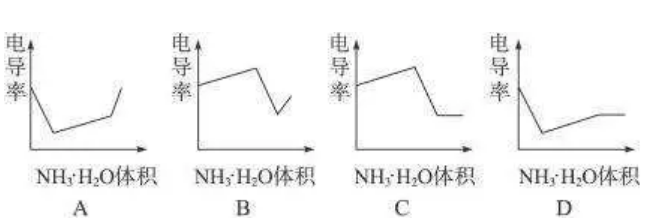
参考答案:D
【点评】氯化氢是强电解质,醋酸是弱电解质,滴加的弱电解质先和氯化氢反应生成强电解质氯化铵,离子数不变,但溶液体积不断增大,溶液被稀释,所以电导率下降;当氯化氢完全被中和后,一水合氨继续电离与弱电解质醋酸反应生成强电解质醋酸铵,所以电导率增大;醋酸也完全反应后,继续滴加氨水,因为溶液被稀释,电导率有下降趋势,故选D.
【典例3】
电导率是衡量电解质溶液导电能力大小的物理量,根据溶液电导率的变化可以确定中和滴定的终点。某化学小组同学利用该原理在常温时,分别测定NaOH溶液和氨水的物质的量浓度,并得到如图所示曲线。
①待测溶液:100 mL 氨水、100 mL NaOH溶液
②标准溶液:0.40mol/L 的盐酸。
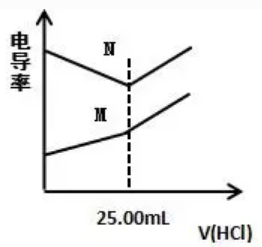
下列说法错误的是( )
A.滴定NaOH溶液时,所得的曲线是B
B.达到滴定终点时,N点溶液的pH<7,M点溶液的pH=7
C.氨水溶液的物质的量浓度为0.10mol/L
D. NaOH溶液的pH=13
参考答案:BD

- 全部评论(0)
(824202736) 评论 href="/plus/view.php?aid=17508">四类中和滴定的电导:最后一道例题考虑水解的话B应该是错的
(15378918863) 评论 href="/plus/view.php?aid=17508">四类中和滴定的电导:最后一道例题的答案是不是只有B啊……我觉得D蛮对的……
(1114820986) 评论 href="/plus/view.php?aid=17508">四类中和滴定的电导:其实第二和第四种情况从0到M点滴定终点时离子数目N和溶液体积
(1295157234) 评论 href="/plus/view.php?aid=17508">四类中和滴定的电导:不错,讲的很明白。
